Chang Sheuw Tian Ran Ling Yao (CS) diformulasikan dari 4 bahan herbal. Formulasi ini telah diuji di berbagai lembaga medis dan akademik dengan hasil yang meyakinkan. Hasil terobosan ini didukung dengan proses ekstraksi yang terkontrol, higienis, dan sangat efektif sangat memungkinkan menjadi alternatif alami yang dapat dipercaya secara ilmiah untuk memperoleh kesehatan secara menyeluruh dan obat pada kanker.
Acute Toxicity Testing
PHARMACOLOGY & TOXICOLOGY LABORATORY, FACULTY OF PHARMACY, UNIVERSITAS GADJAH MADA, YOGYAKARTA, 2001.
Aim of study
- To test the potential acute toxicity of capsules Chang Sheuw Tian Ran Ling Yao expressed as LD50 dose.
- Mode of death during pre-clinical testing.
- Understanding the spectrum of toxic effects (if any)
Research Team
Dr. Imono Argo Donatus, SU., Apt
Arief Nurrochmad, S.Si., Apt
Arief Rahman Hakim, S.Si., Apt
drh. Retno Murwant
Test result
NUMBER OF RATS THAT DIE (%) RESPONSE 14 DAYS AFTER ORAL ADMINISTRATION OF CHANG SHEUW TIAN RAN LING YAO CAPSULE SUSPENSION
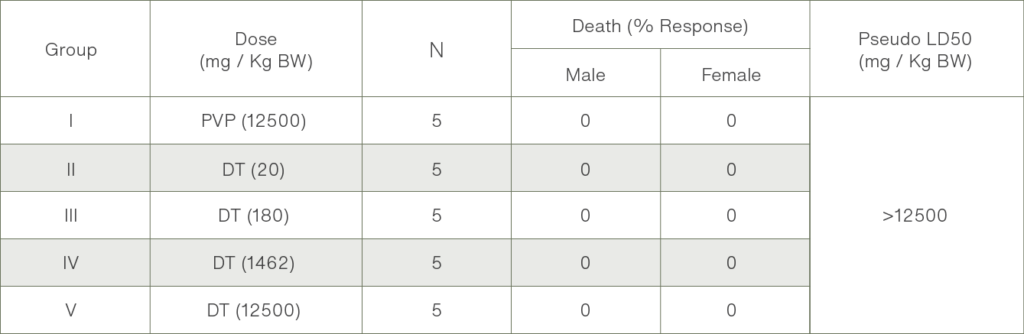
Table 1: Examination results of Acute toxicity Potency (death response) based on administration of dose (LD50).
Up to the 14th day post-administration of the Chang Sheuw Tian Ra Ling Yao Capsule suspension by oral at a dose of 20-12500 mg/Kg BW, no death of male or female mice was recorded (% Response = 0).
Gross description of pathology and several important organs of male rats on the 14th day after administration of a single dose by oral suspension of Chang Sheuw Tian Ran Ling Yao Capsules
Table 2: Examination results of Acute Toxicity Potency towards vital organs based on administration of dose (LD50).
n: Normal | Pa: Lungs | Jan: Heart | Ha: Liver | Lim: Spleen | Gin: Kidney | Lam: Stomach | Us: Intestines
Results Summary
- Extract Chang Sheuw Tian Ran Ling Yao on doses of 20 – 12,500 mg/kg did not reveal any signs of toxicity or mortality in any animal, during the 14 days observation period. No death was recorded (% response = 0).
- The maximum dosing of extract Chang Sheuw Tian Ran Ling Yao (12,500 mg/kg BW), does not cause death.
- The gross pathological examination results up to a dose of 12,500 mg/kgBW did not show any impairment in the vital organs of mice.
- The results of this study suggest that extract Chang Sheuw Tian Ran Ling Yao, does not show any significant spectrum of toxic effects, Hence, should be considered as non-toxic and safe for medicinal use.
Conclusion
In conclusion, the acute toxicity study on extract Chang Sheuw Tian Ran Ling Yao suggests that a dose up to 12,500mg/kg BW (Highest variable) is devoid of any adverse effects in mice. Hence, this study suggests that this study suggest that extract Chang Sheuw Tian Ran Ling Yao should be considered as non-toxic and safe for medicinal use.
Pre-clinical Testing. Carcinogenesis inhibition in Mice
PHARMACOLOGY & TOXICOLOGY LABORATORY, FACULTY OF PHARMACY, UNIVERSITAS GADJAH MADA, YOGYAKARTA, 2002.
Effect of Extract Chang Sheuw Tian Ran Ling Yao (CSTRLY) on BENZO[a]PYRENE Induced Lung Carcinogenesis in the Initiation and Post Initiation Phases in Mice
Aim
To determine the effect of Chang Sheuw Tian Ran Ling Yao (CSTRLY) extract on the inhibition of lung tumour growth in initiation and post initiation phases in mice induced by benzo(a)pyrene (B[a]P) and dimethylbenz(a) anthracene (DMBA).
Method
This study was conducted using the Newborn Mice method using Balpc strain mice (Mus Musculus). Newborn mice were divided into eight groups, each consisting of 10-15 mice. The first group was injected with B[a]P dissolved in dimethylsulfoxide (DMSO) intraperitoneally on the 1st, 8th, and 15th day after birth with doses of 0.2µmol, 0.4µmol, and 0.8µmol respectively.
Research Team
Prof. Dr. Ahmad Fudholi, D.E.A., Apt
Drs. Edy Meiyanto, M. Si., PHD, Apt
Drh. Retno Murwanati
Ganti Winarno Putra
Andi Eviyanti. T
Test Results
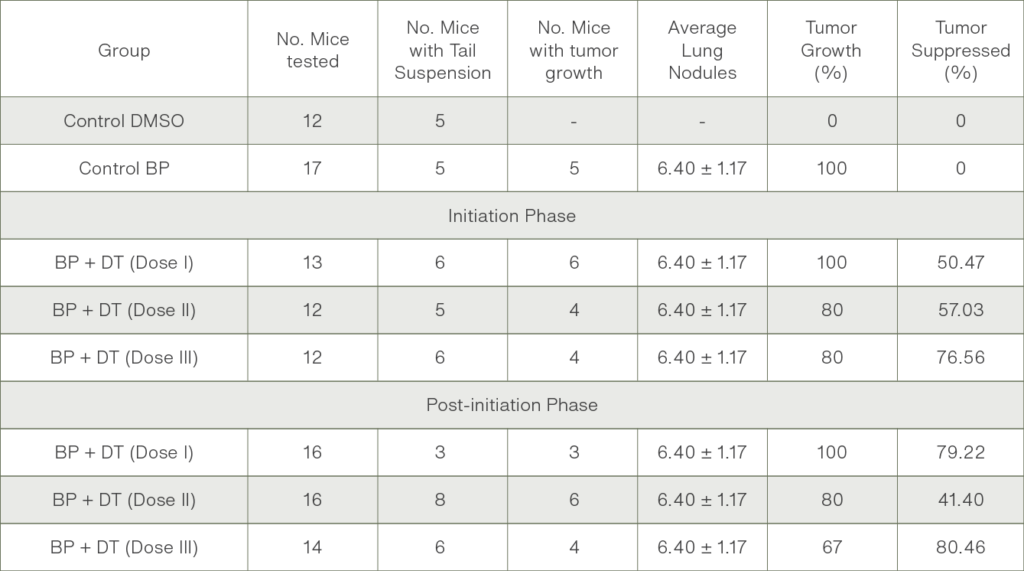
Results Summary
The Administration of extract Chang Sheuw Tian Ran Ling Yao once every 3 days for 30 days after birth revealed reduction on the percentage of mice affected by tumours, and the percentage of tumour growth in the lungs of mice induced by the carcinogen benzo(a)pyrene.
Initiation Phase
Extract with Dose I (450 mg/kg BW) revealed a reduction of tumour growth by 50.47%
Extract with Dose I (450 mg/kg BW) revealed a reduction of tumour growth by 79.22%
Extract with Dose II (750 mg/kg BW) revealed a reduction of tumour growth by by 57.03%
Post-Initiation Phase
Extract with Dose II (750 mg/kg BW) revealed a reduction of tumour growth by 41.40%
Extract with Dose III (1500 mg/kg BW) evealed a reduction of tumour growth by 80.46%
Extract with Dose III (1500 mg/kg BW) revealed a reduction of tumour growth by 76.56%
Conclusion
This study revealed that the herbal extract of Chang Sheuw Tian Ran Ling Yao conveyed the potential to reduce the incidence of tumours induced by carcinogen administration, both B[a]P and DMBA. Moreover, extract Chang Sheuw Tian Ran Ling Yao is also revealed the inhibition of the development of lung carcinogenesis in mice, especially in the early post-initiation phase.
General conclusion
- All mice that were given B[a]P and DMBA on days 1, 8 and 15 after birth revealed tumour growth in their lungs.
- Small carcinogenesis nodules were detected, (yellowish or slightly cloudy white, 0.5 – 1.0 mm in diameter)
In-Vitro Suppression Efficacy Testing of Cancer Cells (Cancer Cell Line)
TISSUE CULTURE LABORATORY RESEARCH AND DEVELOPMENT INSTALLATION, RUMAH SAKIT KANKER DHARMAIS, 2006
Aim
To investigate in-vitro, the potential of carcinogenesis inhibition through cytotoxic and apoptotic activity (cell line) on 4 types of cancer cells:
- Cervical Cancer Cells (CaSki cell line)
- Colon Cancer Cells (HT-29 cell line)
- Breast Cancer Cells (MCF-7)
- Cells Oral Cancer (KB cell)
Observing the mechanism of apoptosis through flow cytometry method using vibrant apoptosis kits
Authorized
Dr dr Abidin Widjanarko, SpPD, KHOM
Director of Human Resources and Education, Rumah Sakit Kanker Dharmais
Test Results
Correlation between Apoptotic process in: Cervical Cancer Cell (CaSki), Colon Cancer Cell (HT-29), Breast Cancer Cell (MCF-7), and Oral Cancer Cell (KB Cell) and the Administered concentration of Chang Sheuw Tian Ran Ling Yao
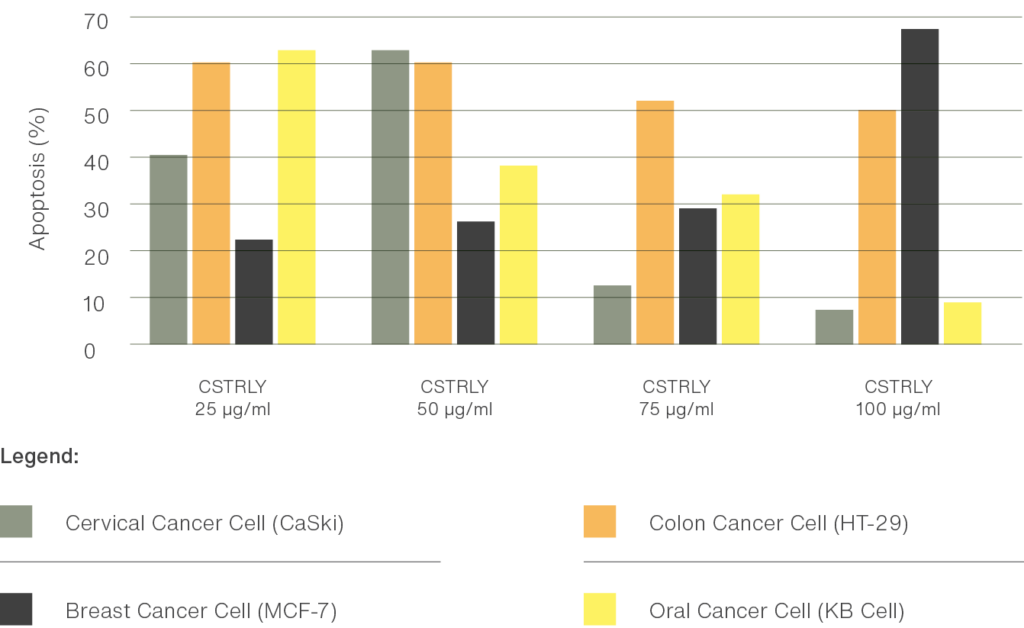
Results Summary
From the results of the study the IC50 value of Chang Sheuw Tian Ran Ling Yao’s ethanol extract was 50μg/ml for cervical cancer cells, 25μg/ml for colon cancer cells, 100μg/ml for breast cancer cells and, 25μg/ml for oral cancer cells after 24 hours of incubation. Correlation analysis between concentration and percentage of cancer apoptosis was 61.95% for cervical cancer cells, 63.73% for colon cancer cells 68.42% for breast cancer cells and 65.12% for oral cancer cells at α = 0.05.
These results show two things as follows: The ethanol extract of Chang Sheuw Tian Ran Ling Yao has a high cytotoxic effect on cervical cancer cells, colon cancer cells, and oral cancer cells. While possessing a high potential to influence a high rate of apoptosis in oral cancer cells, colon cancer cells, breast cancer cells, and oral cancer cells.
Conclusion
The ethanol extract of Chang Sheuw Tian Ran Ling Yao, has a high cytotoxic effect in inhibiting the growth of cervical cancer cells (CaSki line), colon cancer cells (HT-29) and oral cancer cells (KB), but has a low cytotoxic effect on breast cells (MCF-7). However, The ethanol extract of Chang Sheuw Tian Ran Ling Yao, has a high potential to affect the process of apoptosis in breast cancer cells (MCF-7) and oral cancer cells (KB cell). Hence, this study suggests that ethanol extract of Chang Sheuw Tian Ran Ling Yao has high potential of inducing high rates of cytotoxic and apoptotic activities in combating the four investigated cancer cell lines.
Acute Toxicology Testing
PHARMACOLOGY & TOXICOLOGY LABORATORY PHARMACOLOGY & CLINICAL PHARMACY SECTION, FACULTY OF PHARMACY, GADJAH MADA UNIVERSITY, YOGYAKARTA, 2007.
Aim
To determine the potential for acute toxicity which is expressed as the mid-lethal dose range (LD-50), the mode of death during pre-clinical testing, and to understand the spectrum of toxic effects (if any).
Research Team
Protector: Dean of the Faculty of Pharmacy UGM, Yogyakarta, Dr. Marchaban, DESS, Apt
Person responsible: Head of Pharmacology & Toxicology Laboratory, Faculty of Pharmacy UGM,
Yogyakarta, Prof., Lukman Hakim, MSc, PhD, Apt
Member: Dr. Nurlaila, MSi, Apt, Purwantining, MSi, Apt
Test result
- Up to the 15th day after administration of NUTRIGOLD D capsule suspension orally, from the lowest dose to the highest (312 – 6250.23 mg/kg BW), No death was recorded (% response = 0).
- Physical observation of toxic symptoms during the first 3 hours until day 15, administration of NUTRIGOLD D with the lowest dose to the highest dose (6250.23 mg/kg BW in female mice did not show toxic effects, both the central nervous system and somatomotor, autonomic nervous , respiratory, cardiovascular, genitourinary gastrointestinal tract, mucous membranes and eyes.
- The results of macroscopic gross examination of pathology up to the highest dose of 6250.23 mg/kg BW on the vital organs of NUTRIGOLD D administration did not show significant deviations (compared to controls).
Conclusion
Acute toxicity potential – oral NUTRIGOLD D in female mice is included in the “relatively harmless” category.
Pre-clinical Testing for ND-60
PHARMACOLOGY & TOXICOLOGY LABORATORY PHARMACOLOGY & CLINICAL PHARMACY SECTION, FACULTY OF PHARMACY, GADJAH MADA UNIVERSITY, YOGYAKARTA, 2007.
Aim
This study aims to conduct a Preclinical Test of NUTRIGOLD D as an antidiabetic through the understanding of the effect of NUTRIGOLD D on serum glucose levels of hyperglycemic male white rats (Sprague Dawley). While also observing the pancreatic function for both Diabetes Mellitus type I and II.
Research Team
Protector: Dean of the Faculty of Pharmacy UGM, Yogyakarta, Dr. Marchaban, DESS, Apt
Person responsible: Head of Pharmacology & Toxicology Laboratory, Faculty of Pharmacy UGM,
Yogyakarta, Prof., Lukman Hakim, MSc, PhD, Apt
Member: Arief Rahman Hakim, MSi., Apt, Nunung Yuniarti, S.F., MSi., Apt
Conclusion
The administration of NUTRIGOLD D at a dose of 604.8 mg/kg BW and 1209.6 mg/kg BW have the potential to reduce serum glucose levels in male Sprague Dawley mice with pathological conditions resembling Diabetes Mellitus type I, however, the process is deemed to be a long term medication.
Consult our specialists for more product information, treatment plan, suggested diets, and any burden you need to share.
We strive to provide the best palliative care for cancer treatment, let us be by your side through your journey